The 150th anniversary in 2019 of the periodic table of elements proposed by Dmitri Mendeleev is the perfect opportunity to look back on the history of four specific elements. What do radium, polonium, actinium, and lutetium all have in common? They were all discovered by ESPCI scientists or alumni. Let’s revisit that period of intense activity, one equally marked by controversy and the race to discovery.
In 1869, Dmitri Mendeleev published his famous table of periodic classification, which still forms the basis for learning chemical elements today. However, there were fewer known elements at the time, and Mendeleev’s classification provided space for elements with calculable mass to be added. Indeed, his work followed on from over two centuries of attempts by the scientific community to understand and identify different elements and their structures. In the succeeding fifty years, the last twenty-five natural elements were identified. The discovery stories of four among them are closely connected to the history of ESPCI: radium and polonium, discovered by Pierre and Marie Curie (assisted by Gustave Bémont); actinium, discovered by André-Louis Debierne; and finally lutetium, discovered by Georges Urbain.
The discovery of radium and polonium
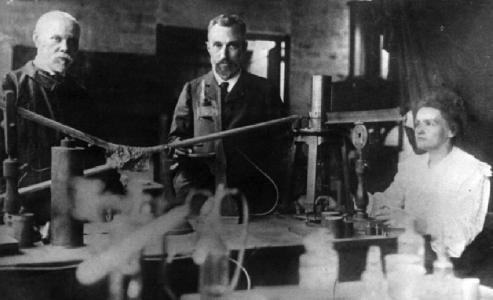 Marie Curie, Pierre Curie and Gustave Bémont in front of the experiment to measure radioactivity at ESPCI around 1898.
Marie Curie, Pierre Curie and Gustave Bémont in front of the experiment to measure radioactivity at ESPCI around 1898.
In 1987 Marie Curie began a thesis on the study of uranic rays recently discovered by Henri Becquerel. Using quartz piezoelectric measurement devices designed by Pierre Curie, she succeeded in precisely measuring the radiation emitted by certain materials, in particular uranium, then thorium. She was able to obtain a measurement of the activity of the latter, which depends on the element itself, but also the quantity present. She also tested many other elements, which proved to be inactive.
Certain measurements carried out on ore like uraninite revealed radiation levels superior to that of uranium (and thorium). From this Marie Curie deduced that the ore was not pure and that another element was hidden within the impurities.
With Pierre’s help, she brought uraninite from uranium mines in Bohemia, and together they tried to extract the elements of interest in a workshop made available by the Ecole Municipale de Physique et de Chimie Industrielles (i.e. the City School of Industrial Physics and Chemistry, which later became ESPCI Paris), where Pierre Curie taught. After several months of purifications and separations, the Curies managed to isolate a substance 400 times more active than uranium. The scientists named it polonium in tribute to Marie Skłodowska-Curie’s native country, and Henri Becquerel presented their work to the Académie des Sciences in July 1898.
During their research on polonium, the Curies also identified a second substance that would be far better characterized: radium. It would be several years before polonium’s atomic mass was known, but radium’s was determined using purity measurements carried out by Eugène Demarçay, a spectrography specialist at the time. Several chemists helped the team purify the ore and extract solutions of radiferous barium chloride. Among them was Gustave Bémont, supervisor of chemistry work at E(S)PCI (who also signed the note presented by H. Becquerel at the Académie des Sciences), and André Debierne, a PC engineer from the ninth graduating class, then working at the Sorbonne’s Chemistry-Physics laboratory.
Actinium discovered on the heels of radium and polonium

André-Louis Debierne, around 1904 On the advice of Marie and Pierre Curie, André Debierne began investigating whether other radioactive materials were present in the ore samples, particularly elements “whose acidic solutions do not precipitate with sulphuretted hydrogen, and precipitate completely with ammonia or ammonia hydrosulfide.” Debierne primarily obtained iron oxide and alumine, but also small quantities of other metals and rare earth elements. He isolated a selection of elements that was radioactive despite the absence of polonium and radium, with activity roughly 100,000 times greater than that of radium—with one particularity: it was not spontaneously luminous.
He presented these discoveries for the first time at the Académie des Sciences in 1899, then in April 1900, when he suggested the name actinium, thorium’s neighboring element.
Georges Urbain and rare earth elements

Georges Urbain. The top student in E(S)PCI’s ninth graduating class, Georges Urbain defended his thesis in 1899 on “Research into the Separation of Rare Earth Elements.” Specifically, he studied yttric earth elements (as opposed to ceric earth elements, another sub-group of the rare earth family) continuously from 1895 until the start of World War One. At the time, only gadolinium appeared in the international table of atomic weights, which gave chemical elements visibility.
Georges Urbain dedicated a large portion of his work to trying to obtain pure elements from ytterbium discovered by Jean Charles Galissard de Marignac.
He was able to prove that ytterbium is composed of two substances. He then proceeded to carry out a spectral analysis of both elements for their full characterization and demonstrate that they are distinct elements. In 1907 Albin Haller presented his notes to the Académie des Sciences, where he suggested the new element, produced from the division of ytterbium, be called lutecium in homage of the city of Paris.
Controversy then broke out as to the ownership of the discovery, with Carl Auer Von Welsbach claiming to have discovered the substance earlier and proposing the name cassiopeium. In the end, the International Commission on Atomic Weights ruled in favor of Urbain, but imposed the spelling lutetium.
In 1911 Urbain isolated a new element in his samples and called it celtium, but his work was interrupted by the war. He continued his research when the conflict was over, and announced his new discovery in 1922. He characterized celtium through a complete study of its emission spectrum, but he mistakenly placed it in the rare earth elements column. George de Hevesy and Dirk Coster, collaborators of Niels Bohr, also characterized it, placing it closer to zirconium and calling it hafnium (in homage to the former name of Copenhagen). What followed was a controversy that lasted several decades, during which the two names were used simultaneously, until finally the name hafnium won the day.
 G. Urbain degree of ESPCI. Gift from the family to the school. © G. Durey
G. Urbain degree of ESPCI. Gift from the family to the school. © G. Durey
References:
Minutes from the Académie des Sciences, November 4, 1907
https://gallica.bnf.fr/ark:/12148/bpt6k3099v/f759.item
Minutes from the Académie des Sciences, December 26, 1898
https://www.academie-sciences.fr/pdf/dossiers/Curie/Curie_pdf/CR1898_p175_178.pdf
Minutes from the Académie des Sciences, October 16, 1899
https://gallica.bnf.fr/ark:/12148/bpt6k3085b/f593.item
Minutes from the Académie des Sciences, April 1900 https://gallica.bnf.fr/ark:/12148/bpt6k3086n/f906.image
Note on the life and work of Georges Urban, lecture given by Robert Courrier at the Académie des Sciences on December 11, 1972.
https://www.academie-sciences.fr/pdf/eloges/urbain_notice.pdf
Reports presented at the international physics congress in Paris in 1900 hosted by the Société Française de Physique (French Society of Physics).
Volume III, Electro-optique et ionisation, applications, physique cosmique, physique biologique, collected and published by Ch.-Éd. Guillaume and L. Poincaré,...
https://gallica.bnf.fr/ark:/12148/bpt6k9813472t/f102.item.r=actinium
To learn more about Georges Urbain, see the bibliographic note created by ESPCI Alumni: https://drive.google.com/file/d/0B_PVTM3Gqf1-VkZsRXMxSU9XaDQ/view